Why electroclean?
Electrocleaning before plating is necessary to remove oils, grease, rouge, passive oxide films, and other surface contaminants left over from the manufacturing and polishing processes.
Electrocleaning jewelry utilizes a heated alkaline solution, low voltage direct elect current and a stainless steel anode. The piece to be cleaned is connected to the cathode side (-) of the rectifier giving it a negative charge, using a stainless steel anode connected to the positive (+) lead of the rectifier, low voltage current is applied producing hydrogen at the cathode through electrolysis.
The hydrogen bubbles scrub the piece and the contaminants are dissolved into the solution. (Note: Spring steel can be embrittled by hydrogen so an alternative process my be necessary.)
This process is followed by a thorough rinse in clean deionized (or distilled) water followed by a 20-30 second acid dip. The acid removes film left behind by the alkaline cleaner and activates the surface for plating. A simple and effective acid dip can be prepared using Sparex at 2.5 ounces per quart or 70 grams per liter (one-quarter it’s recommended pickling strength). And remember, NEVER steam a piece during the cleaning process.
electro-spar-tivaThe acid dip is then followed by a 2nd distilled water rinse to remove the acid before plating. (A double rinse is recommended if plating with a cyanide based solution.) The acid dip, also called neutralization, prevents the drag out of the electrocleaner into the plating solution, avoiding contamination and prolonging the life and quality of the plating solution. This also “activates” the surface of the metal prior to plating.
The rinse water should not bead on the piece after activation. Water should sheet off the piece. Any beading indicates an incomplete cleaning process. If beading occurs, repeat the electrocleaning steps above, change to a fresh electrocleaning solution, or consider using a different electrocleaner.
Electroplating is a process where each step reliant on the prior step, and is necessary to consistent and quality plating.
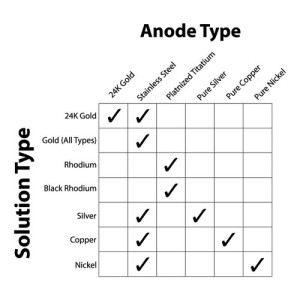
Click here for a Guide to Electro-Plating Chart
Article Source: [http://www.krohnindustries.com/why-electroclean/]: [April 25, 2016]







Leave A Comment